The phosphatidylinositol 3-kinase (PI3K) protein family regulates cellular functions including growth, proliferation, survival, differentiation, and motility. Because these functions are critical for cancer progression, many companies are developing therapeutic compounds to inhibit PI3K. GW inventors identified an alternative splicing variant of PIK3CD that causes insensitivity to one or more of these PI3K inhibitors. This discovery can be developed into a companion diagnostic test that will limit PI3K inhibitor treatment to patients that can benefit from it.
Additional PI3K inhibitors can be screened in cell-based assays to determine whether the splicing variant of PIK3CD is insensitive to more drugs. Conversely, cell-based assays can be used to find drugs that still inhibit PI3K produced by splicing variants of PIK3CD. The splicing variant ofPIK3CD can be readily detected by testing of mRNA from a tumor sample.
Abnormal alternative splicing of several oncogenes and tumor suppressor genes has been identified in cancers. The GW inventors identified 12 differentially spliced genes that contribute to oncogenic signaling pathways. These splicing variants represent targets for developing additional companion diagnostics and compound screening assays. In vivo data is in hand for PIK3CD companion diagnostic.
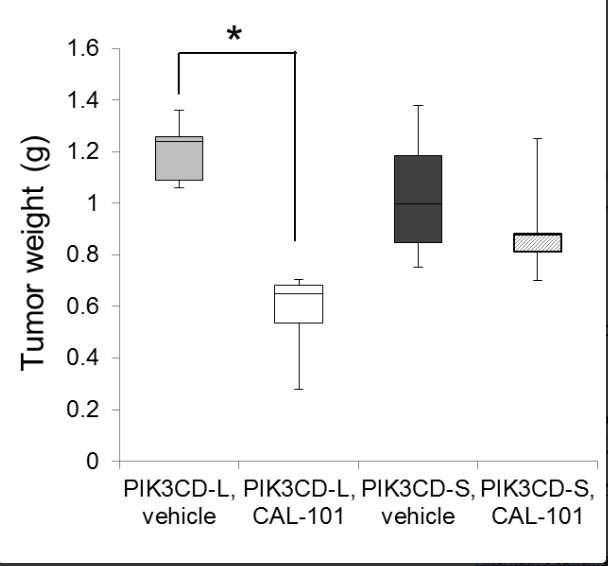
Figure: Tumor Weight in vivo - Short form resistant to PIK3CD inhibitor.
Applications:
- Personalize treatment with PI3K inhibitors
- Avoid treating cancer patients that will not respond to your drug
- Improve clinical trial outcomes
- Screen for PI3K inhibitors with efficacy against splicing variants
- Develop additional companion diagnostics for cancer based on other splicing variants
Advantages:
- Simple mRNA or cDNA genetic test to stratify patients
- Cell-based screening assay to test PI3K inhibitors against splice variants