Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is one of the deadliest infectious diseases. Emergence of drug resistant strains of Mtb and co-infection with HIV has made TB both difficult and expensive to treat. New TB therapies are needed to shorten treatment and be effective against all strains and metabolic states of the organism. Mtb synthesize isoprenoids (essential for the bacterial cell wall biosynthesis) via the nonmevalonate pathway, which is absent in humans. Therefore, the bacterial enzymes can be specifically targeted without interfering with human metabolism, making this an attractive pathway in the search for novel drugs. Development of inhibitors of 1-deoxy-D-xylulose-5-phosphate reducto-isomerase (Dxr), an essential enzyme for Mtb isoprenoid synthesis, is a novel approach toward the development of a new TB chemotherapy. Natural product, fosmidomycin inhibits Dxr, kills other organisms dependent on this enzyme, but is not effective against Mtb.
Researchers at the George Washington University have synthesized and tested novel inhibitors of Dxr that are analogs of fosmidomycin and its derivative, FR900098. These novel compounds are distinct from the known analogs because of their ability to target two major binding sites in Dxr, yet retain high affinity as ligands. Select compounds from two distinct medicinal chemistry strategies are effective at very low concentrations in killing Mtb and other bacteria.
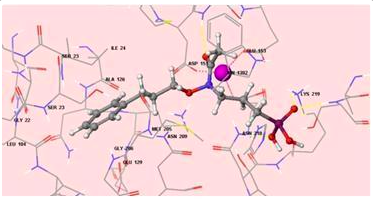
Fig. 1 - Docking representation of a potential inhibitor

Fig. 2 - Mtb Dxr active site with fosmidomycin and NADP+
Applications:
- Selected compounds are selective inhibitors of bacterial 1-deoxy-D-xylulose-5-phosphate reducto-isomerase (Dxr)
- These select compounds can be developed into treatment for TB and other bacterial infections.