The withdrawal of drugs from the market is a costly consequence of undetected adverse effects or toxicity. Over 30% of such withdrawals are due to cardiac toxicity (pro-arrhythmic effects mediated by cardiac ion channels), therefore regulatory agreements mandate the testing of all new drugs for cardiac liability.
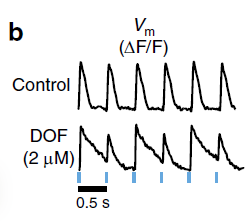
GW researchers developed the first scalable automated all-optical system for high-throughput cardiac electrophysiology and electromechanics. The system includes a method for combined optical pacing and optical recording (what we term all-optical electrophysiology) from a variety of primary and stem-cell-derived cells and tissues. Notably, the system does not require any genetic modification of the cells of interest and can be used with low power red light LEDs. This platform allows for active dynamic interrogation, such as robust pacing protocols that can reveal Vm, [Ca2+]i or contraction’s frequency response (restitution) and temporal instabilities (Fig. 1).
Applications:
1. High-throughput screening for cardiac liability
2. Phenotyping iPSC-derived cardiomyocytes
Advantages:
1. Fast, cost-effective, high-throughput (>600 samples/hour)
2. Does not require genetic modification of cells of interest
3. Measures Vm and [Ca2+]i simultaneously
4. Safer for cells due to low intensity and long wavelength light used
5. Identifies cardiotoxicity before resources are wasted on clinical trials
6. Compatible with standard 96- or 384-well plates