The tumor microenvironment (TME) plays a key role in tumor growth and survival. Often the immunosuppressive nature of the TME prevents success of treatments including immune checkpoint blockade (ICB), radiation therapy (RT), and chemotherapy. Macrophages in the TME contribute to its immunosuppressive or immunostimulatory character. M2 macrophages secrete anti-inflammatory cytokines, such as transforming growth factor (TGFβ) and interleukin (IL-10), associated with cancer, angiogenesis, tumor invasion, and migration. On the contrary, M1 macrophages secrete pro-inflammatory cytokines such as interleukin (IL-12) and tumor necrosis factor (TNFα) and have an anti-tumor function. M1/M2 ratio serves as a prognostic indicator of the progression of tumors. A decrease of M2 macrophages over M1 macrophages reduces the chance of tumor recurrence.
Our inventors designed a method of decreasing M2 by treating naive and/or M1 macrophages ex vivo with an inhibitor of Histone Deacetylase 6 (HDAC6i). The resultant HDAC6i-activated macrophages with elevated immunostimulatory cytokines are then administered to the patient. HDAC6i-activated macrophages reduced tumor size significantly in a mouse model of melanoma. While many HDAC6i can be toxic to patients, in this case HDAC6i toxicities are avoided by administering only in culture to the macrophage rather than to patients themselves.
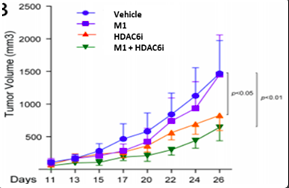
Figure: In mice, M1+HDAC6i shows a significant decrease in melanoma tumor size compared to Vehicle or untreated M1.
Applications:
- Cell therapy for cancer, preferably solid tumors
Advantages:
- Increases immunostimulatory response of macrophages
- Remodels the tumor microenvironment to be less immunosuppressive
- Enhances other treatments, such as immunotherapy and radiation therapy
- Avoids negative impact of HDAC6i toxicity to patients