Researchers at The George Washington University are developing a fast label-free mapping system of excitation-contraction-metabolic waves at hundreds to thousands (e.g., 100-5,000) of frames per second for mapping cardica physiology. Such a system allows for clinical mapping of atrial and ventricular arrhythmia during, or prior to, ablation procedures to guide such procedures through the identification of the source/driver or sources/drivers of atrial tachycardia or fibrillation and of ventricular tachycardia or fibrillation.
The system has a number of benefits and advantages. First, a fast label-free method for mapping cardiac physiology has the ability to better detect focal sources of arrhythmias as well as reentrant pathways with high spatio-temporal resolution. Second, this method and system is unique in that it can be stand-alone for cardiac electrical dysfunction detection purposes, but it can also be integrated onto an organ conformal electronics platform for simultaneous optical mapping and ablation purposes. Third, this system and method is free of labels, is non-toxic to the heart (and therefore the patient), and it capitalizes on the intrinsic fluorescence of metabolic signals. Fourth, metabolic waves are captured on a beat-to-beat basis, making this technology useful for readily replacing optical mapping of cardiac electrophysiology in experimental settings without the need for exogenous fluorescent dyes. Fifth, this system and method provides direct evaluation and informs about pathological metabolic states, which allows dynamic mapping of ischemic, hypoxic and other pathological metabolic events leading to electromechanical dysfunction and lethal arrhythmia and heart failure.
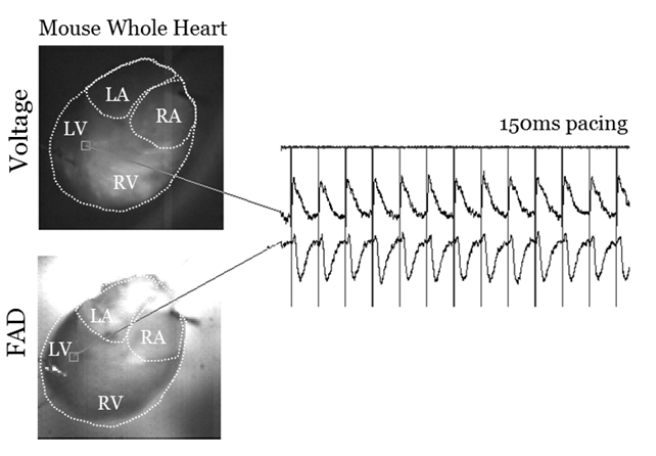
The system and method have been established in whole ex vivo mouse hearts, isolated mouse and rat atrial preparations, and is being tested ex vivo in human heart. The spatio-temporal optical mapping recordings of metabolic transients are correlative with electrical conduction maps on a beat-to-beat basis. A small conformal device can be provided to quickly detect label-free metabolic waves of cardiac physiology over a wide range of mammalian hearts. For example, conformal devices such as then ones described in US20180235692A1. In addition, as described in US20180235692A1, an ablation catheter can be integrated within the same conformal flexible/stretchable bioelectronics device that will simultaneously sense and report the source or sources of tachycardia or fibrillation. In addition, an actuator can be provided to impart an electrical correction to the heart rhythm, such as an electrical pulse or shock.
Applications:
- Clinical mapping of atrial and ventricular arrhythmia during, or prior to, ablation procedures to guide such procedures through the identification of the sources of atrial tachycardia or fibrillation and of ventricular tachycardia or fibrillation.