Previously, GW researchers invented a method to selectively kill cancer cells with cold atmospheric plasma (CAP) while leaving neighboring normal cells intact. In a subsequent study, the researchers showed that similar anti-cancer effects can be achieved indirectly using CAP-stimulated media (CAPSM).
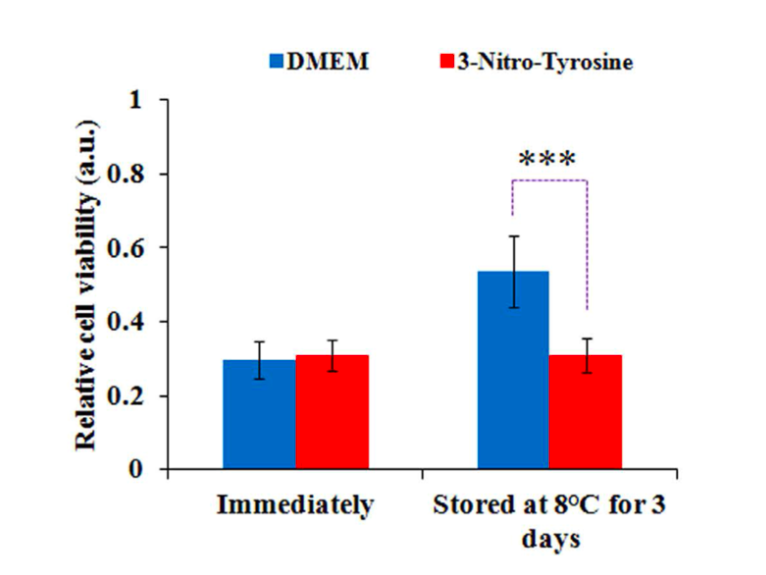
Stability of CAPSM (preservation of its anti-cancer property over time) is a key consideration for its future clinical application. Previously, others have reported that CAPSM needs to be stored at -80ºC (-112ºF) for it to maintain its stability for 7 days. In the current invention, GW researchers demonstrate that stability of CAPSM can be maintained for 3-7 days at 8ºC by using PBS or modified DMEM as the media.
In addition, the current invention identifies a number of stimulation parameters that can affect the anti-cancer capacity of CAPSM. These parameters include (1) plasma treatment time, (2) well size, (3) size of the gap between the plasma source and the media, (4) volume of media, and (5) specific amino acid contents of the media. This invention enables optimization of CAPSM therapy to have high anti-cancer capability. Given that CAPSM may be useful in instances where direct application of CAP to tumor cells or tissues is not feasible, the current invention may lead to a broader application of CAP-based cancer treatments.
The original CAP therapy device has been used on a human patient under a therapeutic use exemption to treat the area where a tumor was surgically removed, in order to kill any tumor cells that may have been left behind and prevent remission. Submission for FDA approval of the CAP device is anticipated in 2016.
Applications:
Cancer therapy
Advantages:
1. Applicable in situations where CAP treatment (direct exposure of tumors to CAP) is not feasible
2. Plasma device can reside at manufacturing facility, instead of in operating room
3. Enables sales of single use product versus expensive device